Latest Info on SO2 Molecular Geometry
Here, we will certainly explain the SO2 Molecular Geometry carefully. Sulphur Dioxide, which is additionally acknowledged as Sulphur Dioxide, is the entity of a bond between Sulfur and Oxygen atoms. It can be viewed as a formula composed of SO2. Right here, we will undoubtedly describe SO2 molecular geometry, SO2 electron geometry, SO2 bond angle, and SO2 Lewis framework.
SO2 Lewis Framework
Before quickly delving into the lewis structure of SO2, allow’s have a short conversation concerning the usefulness of the lewis framework and the actions to draw it.
Lewis structure is the positioning of the electrons around the atoms of a substance. This framework benefits us to learn about the sort of bonds and the number of bonds that form the substance.
1: Identifying the total variety of valence electrons in the particle is the initial and most impressive step. While doing so, do care for the +,– indications. A ‘+’ indicator implies shedding electrons as well as ‘-‘ suggests obtaining.
2: Next point is establishing the central atom. The atom with the widest variety of critical areas is the central atom.
3: The third step is developing a skeleton structure with solitary bonds only.
4: Next, our work is attaining the octet of the atoms with the resting electrons after forming the single bonds. Constantly begin with the electronegative atoms, then bring them to the electropositive ones.
5: Providing double or triple bonds is fundamental if it is needed for satisfying the octet regulation for all atoms.
6: At last, it is essential to examine if all the atoms have their lowest feasible formal fee.
Formal charge calculation can be done making use of:-.
Formal Charge = [no. of valence electrons] — [electrons in only pairs + 1/2 the variety of bonding electrons]
This formula explicitly suggests the connection between the number of bonding electrons and their web link to how many are officially “maintained” by the atom.
For instance, applying this to BH4, we obtain.
The number of valence electrons for boron is 3. The number of non-bonded electrons is absolute no. The total variety of bonding electrons around the boron is 8 (complete octet). One-half of this is 4.
Now let’s see the lewis framework of SO2.
In SO2, the Sulfur’s valence electron = 6.
As well as the valence electrons of oxygen = 6.
There are 2 oxygen atoms in the substance, hence = 6 * 2 = 12.
So, total valence electrons = 18.
Deducting that from the overall valence electrons, we obtain 10 electrons remaining. We need to put these staying electrons around the atoms according to the need.
This will last but not least finish the octet of the atoms. Oxygen has two lone sets as well as Sulfur has one lone pair.
At last, do not fail to remember to verify the legal cost of all the atoms!
SO2 Hybridization & SO2 Molecular Geometry
The hybridization of SO2 is Sp2.
Currently, the hybridization of SO2 can be recognized in 2 methods: the concept and the 2nd is straight applying the formula. I would advise comprehending the theory initially, and afterwards, you can go with the procedure.
A sharp edge for you, when 1 s orbital unites with 2 p orbitals, it acquires in Sp2 hybridization having three equal orbitals.
Too, in case of SO2, the ground state digital configuration is 1s2 2s2 2p6 3s2 3p4. When in a thrilling state, one electron from 3px, activities to 3d orbital. Hence we have 3p3.
The 3s2 and 3p3 connect to create Sp2 hybridization with three comparable orbitals, consisting of 2 combined electrons and two unpaired.
For creating two sigma bonds with oxygen atoms, sulphur needs the two unpaired electrons from the Sp2 hybridized orbitals. Et cetera, two combined orbitals make the lone pair of sulphur.
Considering the various other 2 electrons of 3p which were not associated with hybridization?
Well, those 2 (i.e. among the 3p orbital and one more electron in 3d) created the bonds between sulphur and oxygen. There’s an image connected listed below for better understanding.
Now concerning the formula part.
The formula for finding hybridization of any substance is;
H = 1/2 [V+M- C+A]
Where,
H illustrates Hybridization
V is the no. of valence electrons
M is the matter of monovalent atoms present
C stands for the cationic fee
A represents the anionic fee
Right here, if H is 2, it’s Sp hybridization.
When H = 3, it’s Sp2 hybridization.
When H = 4, it’s Sp3 hybridization.
H = 5, its Sp4 hybridization.
And ultimately, when H is 6, it will undoubtedly be Sp3d2 hybridization.
For SO2, the variety of valence electrons of the S atom = 6 and the type of monovalent atoms = 0, since oxygen is a divalent atom.
Right here, cationic and anionic costs will undoubtedly be 0 as it’s a neutral compound.
Hence, H = 1/2 [6 +0 -0 +0]
H = 1/2 * 6
H = 3 = Sp2 hybridization.
I believe the hybridization of SO2 is clear from both the explained ideas.
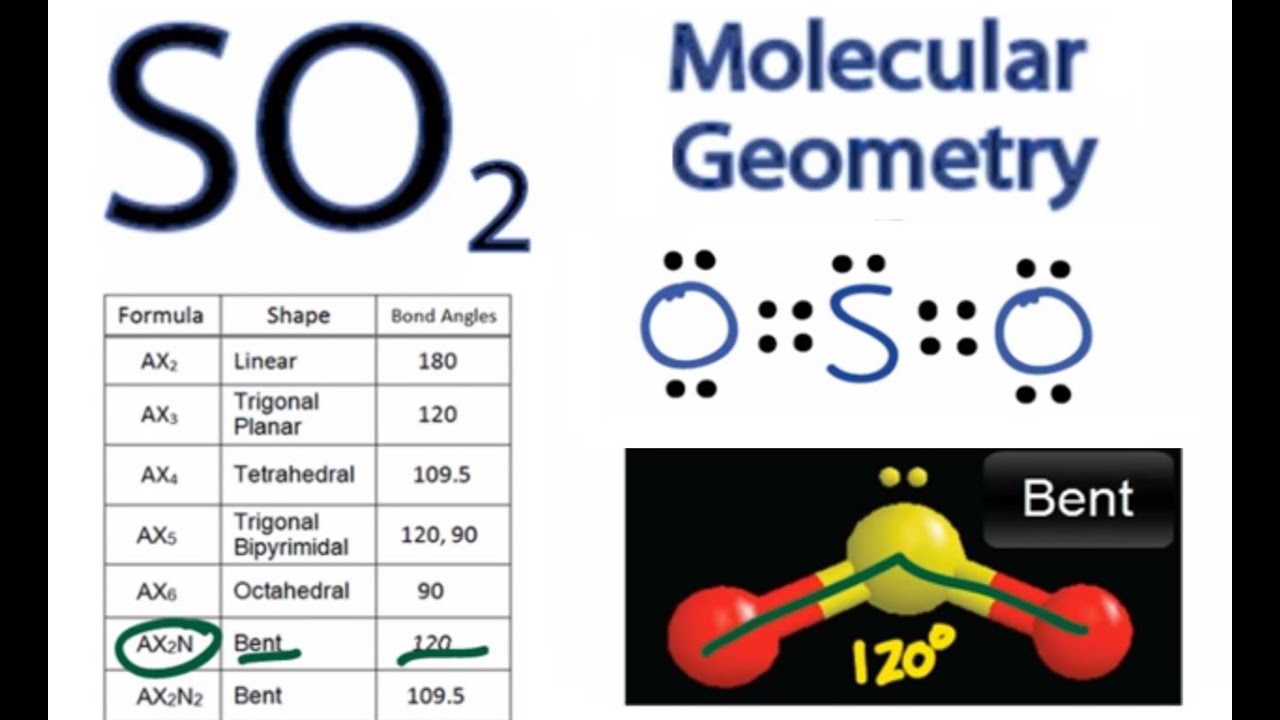
SO2 Molecular geometry
The molecular geometry of SO2 is curved, with a bond angle of 120 °.
Here, A = central atom, X = bordering atoms as well as E = the lone sets. SO2 is an AX2E particle with two surrounding atoms, i.e. oxygen, and one lone pair of Sulfur.
However, the electron geometry of SO2 is trigonal planar. You must be wondering about this brand-new term. Let me explain.
So, electron geometry is different from molecular geometry because it thinks about all the electron pairs (including only sets) while establishing the form. At the same time, molecular geometry takes into consideration only the atoms.
In the absence of a lone pair, both the geometries are the same for any compound.
SO2 Molecular Orbital Diagram
A molecular orbital layout offers us an idea concerning how the atomic orbitals of two various atoms can fuse and generate a brand-new orbital.
This additional helps us discover the bond order, bond size, and bond toughness of any compound.
In this MO, we can see that the AO of Sulfur, on the left-hand side, connects with the AO of oxygen on the right-hand side.
We can see 18 electrons have completed the orbitals with the proper rule.
There is particular non-bonding orbitals present there also. Likewise, the antibonding orbitals are vacant in the circumstance of SO2.
This amounts to the description of the molecular orbital layout of SO2.
Similarities between Sulfur and also Oxygen atoms
Both O as well as S have the same outer electrical arrangement of ns2 and also np4.
O as well as S is usually divalent.
O as well as S are nonmetals.
Both exhibit allopatric types.
In response to steels, both respond with the oxidation state of -2.
While responding with nonmetals, both form covalent substances, such as H2O, H2S, CO2, and CS2.
Dissimilarities between oxygen and Sulphur
Oxygen has two allotropic types, while Sulphur has three allotropic types.
It is gas at standard temperature level, while Sulphur is solid at ordinary temperature.
Also, it is sparingly soluble in water, while Sulphur is not soluble in water.
It aids in combustion while Sulphur is combustible itself.
And, it is paramagnetic, while Sulphur is diamagnetic.
Oxygen does not respond with water, while when heavy steam is gone through boiling sulphur, miniature hydrogen sulphide and SO2 are made.
Oxy does not react with acids, while Sulphur is conveniently oxidized by focused sulfuric acid or nitric acid.
SO2 Preparation
SO2 can be generated in numerous methods.
1– The primary production of SO2 is throughout the manufacture of sulfuric acid utilizing the get in touch with the process. Among all various other means to produce SO2, this technique is extensively used in industries. (Since Chemistry has a lot to do with history, below’s a historical reality for you! In 1979, the United States made use of 23.6 million tonnes of SO2 for manufacturing sulfuric acid.).
2– SO2 can be created by shedding Sulphur or products consisting of Sulphur.
S + O2– > SO2.
2 H2S + 3O2– > 2H2O + 2SO2.
3– SO2 production can likewise be done by the roasting of pyrite, sphalerite, as well as cinnabar (sulphide ores).
4– In the formation of calcium silicate cement, SO2 is generated as a byproduct.
2 CaSO4 + 2SiO2 + C– > 2CaSiO3 + 2SO2 + CARBON DIOXIDE.
5– Busy, the response between hot focused sulfuric acid and copper turnings leads to SO2 development.
Cu + 2H2SO4– > CuSO4 + SO2 + 2H2O.
6– Natural catastrophes like volcanic eruptions can generate a massive amount of SO2.
SO2 Molecular Geometry: Is Polar or Nonpolar?
Sulphur dioxide is polar. The difference in electronegativity between sulphur and oxygen atoms creates polarity in the particle. Oxygen has a better electronegative capacity than sulphur. Consequently, oxygen puts in a lot more pull on the covalent bonds in sulphur dioxide. The part of the molecule that has both oxygen atoms on it is a little adversely charged.
Whereas the portion which has the sulphur atom has a slightly favourable cost. This makes SO2 a polar particle-like H2S. Furthermore, the unbonded electrons on the sulphur and also oxygen create repulsion between particles.
This is another cause of the polarity of the sulphur dioxide particle.
Sulphur dioxide Impacts on Humans
- Sulphur dioxide is a toxic gas well as is directly unsafe for human health and wellness.
- It can aggravate the skin and mucous membrane layers of the eyes, nose, throat, and lungs.
- Its high concentrations can cause inflammation and irritation of the respiratory system.
- Increased emissions of sulphur dioxide airborne can lead to the formation of other sulphur oxides (SOx).
- SOx can react with various other substances in the atmosphere to develop little particles.
- These tiny particles might permeate deeply right into the lungs, and their adequate amount can contribute to health problems.
Verdict
- The molecular geometry of sulphur dioxide is a curved shape.
- Sulphur to the Oxygen ratio in Sulfur dioxide is 1:2.
- Sulphur dioxide molecule has two double bonds between the Sulfur atom and also Oxygen atoms.
- There are five only sets of electrons in the molecule of sulphur dioxide.
- Molar mass of SO2 = 64.066 g/mol.
- SO2 provides a weak acid solution when liquified in water.
- This post describes every little thing you need to understand about SO2.