What Is A Diatomic Element?
Diatomic Elements: Diatomic molecules are molecules composed of only two atoms, of the same or different chemical elements. The prefix di- is of Greek origin, meaning “two”. If a diatomic molecule consists of two atoms of the same element, such as hydrogen (H2) or oxygen (O2), then it is said to be homonuclear. Otherwise, if a diatomic molecule consists of two different atoms, such as carbon monoxide (CO) or nitric oxide (NO), the molecule is said to be heteronuclear. The bond in a homonuclear diatomic molecule is non-polar.
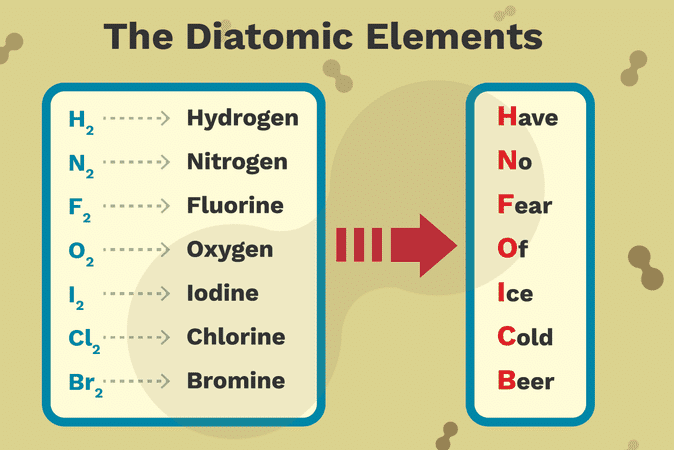
The only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (STP) (or typical laboratory conditions of 1 bar and 25 °C) are the gases hydrogen (H2), nitrogen (N2), oxygen (O2), fluorine (F2), and chlorine (Cl2).
The noble gases (helium, neon, argon, krypton, xenon, and radon) are also gases at STP, but they are monatomic. The homonuclear diatomic gases and noble gases together are called “elemental gases” or “molecular gases”, to distinguish them from other gases that are chemical compounds.
At slightly elevated temperatures, the halogens bromine (Br2) and iodine (I2) also form diatomic gases. All halogens have been observed as diatomic molecules, except for astatine, which is uncertain.
The mnemonics BrINClHOF, pronounced “Brinklehof”, and HONClBrIF, pronounced “Honkelbrif”, and HOFBrINCl (pronounced as Hofbrinkle) have been coined to aid recall of the list of diatomic elements.
What are the 8 diatomic elements?
The elements found as diatomic molecules are hydrogen (H, element 1), nitrogen (N, element 7), oxygen (O, element 8), fluorine (F, element 9), chlorine (Cl, element 17), bromine (Br, element 35), and iodine (I, element 53).
What are the 7 types of diatomic elements?
7 Diatomic Elements
This is a list of the seven diatomic elements. The seven diatomic elements are:
- Hydrogen (H2)
- Nitrogen (N2)
- Oxygen (O2)
- Fluorine (F2)
- Chlorine (Cl2)
- Iodine (I2)
- Bromine (Br2)
All of these elements are nonmetals, since the halogens are a special type of nonmetallic element. Bromine is a liquid at room temperature, while the other elements all gases under ordinary conditions. As the temperature is lowered or pressure is increased, the other elements become diatomic liquids.
Astatine (atomic number 85, symbol At) and tennessine (atomic number 117, symbol Ts) are also in the halogen group and may form diatomic molecules. However, some scientists predict tennessine may behave more like a noble gas.
What Are The Diatomic Elements
Diatomic elements played an important role in the elucidation of the concepts of element, atom, and molecule in the 19th century, because some of the most common elements, such as hydrogen, oxygen, and nitrogen, occur as diatomic molecules. John Dalton’s original atomic hypothesis assumed that all elements were monatomic and that the atoms in compounds would normally have the simplest atomic ratios with respect to one another. For example, Dalton assumed water’s formula to be HO, giving the atomic weight of oxygen as eight times that of hydrogen, instead of the modern value of about 16. As a consequence, confusion existed regarding atomic weights and molecular formulas for about half a century.
As early as 1805, Gay-Lussac and von Humboldt showed that water is formed of two volumes of hydrogen and one volume of oxygen, and by 1811 Amedeo Avogadro had arrived at the correct interpretation of water’s composition, based on what is now called Avogadro’s law and the assumption of diatomic elemental molecules. However, these results were mostly ignored until 1860, partly due to the belief that atoms of one element would have no chemical affinity toward atoms of the same element, and also partly due to apparent exceptions to Avogadro’s law that were not explained until later in terms of dissociating molecules.
At the 1860 Karlsruhe Congress on atomic weights, Cannizzaro resurrected Avogadro’s ideas and used them to produce a consistent table of atomic weights, which mostly agree with modern values. These weights were an important prerequisite for the discovery of the periodic law by Dmitri Mendeleev and Lothar Meyer.
Diatomic Elements List
A diatomic element is a molecule of an element consisting of two atoms. It is a form of homonuclear diatomic molecule. There are only 7 diatomic elements in total and only 5 diatomic elements at standard temperature and pressure (STP).
The following 5 element gases are found as diatomic molecules at room temperature and pressure:
- Hydrogen – H2
- Nitrogen – N2
- Oxygen – O2
- Fluorine – F2
- Chlorine – Cl2
Bromine and iodine commonly exist in liquid form, but also as diatomic gases at slightly higher temperatures, making a total of 7 diatomic elements.
- Bromine – Br2
- Iodine – I2
How To Remember the Diatomic Elements
An easy mnemonic device is:
Have No Fear Of Ice Cold Beer
Hydrogen
Nitrogen
Fluorine
Oxygen
Iodine
Chlorine
Bromine
The diatomic elements are the –ine halogens (fluorine, chlorine, bromine, iodine) and elements with a –gen ending (hydrogen, oxygen, nitrogen). Astatine is another halogen, but its behavior is not known.
What Are Diatomic Elements
Diatomic molecules are normally in their lowest or ground state, which conventionally is also known as the {\displaystyle X} state. When a gas of diatomic molecules is bombarded by energetic electrons, some of the molecules may be excited to higher electronic states, as occurs, for example, in the natural aurora; high-altitude nuclear explosions; and rocket-borne electron gun experiments. Such excitation can also occur when the gas absorbs light or other electromagnetic radiation. The excited states are unstable and naturally relax back to the ground state. Over various short time scales after the excitation (typically a fraction of a second, or sometimes longer than a second if the excited state is metastable), transitions occur from higher to lower electronic states and ultimately to the ground state, and in each transition results a photon is emitted. This emission is known as fluorescence. Successively higher electronic states are conventionally named {\displaystyle A}, {\displaystyle B}, {\displaystyle C}, etc. (but this convention is not always followed, and sometimes lower case letters and alphabetically out-of-sequence letters are used, as in the example given below). The excitation energy must be greater than or equal to the energy of the electronic state in order for the excitation to occur.
In quantum theory, an electronic state of a diatomic molecule is represented by the molecular term symbol
- {\displaystyle ^{2S+1}\Lambda (v)}
where {\displaystyle S} is the total electronic spin quantum number, {\displaystyle \Lambda } is the total electronic angular momentum quantum number along the internuclear axis, and {\displaystyle v} is the vibrational quantum number. {\displaystyle \Lambda } takes on values 0, 1, 2, …, which are represented by the electronic state symbols {\displaystyle \Sigma }, {\displaystyle \Pi }, {\displaystyle \Delta },…. For example, the following table lists the common electronic states (without vibrational quantum numbers) along with the energy of the lowest vibrational level ({\displaystyle v=0}) of diatomic nitrogen (N2), the most abundant gas in the Earth’s atmosphere. In the table, the subscripts and superscripts after {\displaystyle \Lambda } give additional quantum mechanical details about the electronic state.
All Diatomic Elements
Something in the way you ask makes me think you are referring to “Diatomic Homonuclear molecules”, a.k.a -non officially- Diatomic Elements.
Well, some elements are more stable combined with atoms of the same type than alone. So they “prefer” to be attached to another atom of the same element.
Individual atoms are quite reactive because of their incomplete valence shells and by their closeness to their correspondent noble gases. We can say that those atoms really want to complete his shells and that translates in their high electronegativy.
Why? Its just the way nature works. But as we scientists hate empirical answers, I´ll give you additional data, even if they’re no is a Ultimate Why.
*Firs, let’s remember which of all elements are as you call, diatomic:

The fact that these elements are diatomic is ONLY when they are alone, NOT when chemically bonded to another atom. When hydrogen is bonded to something other than itself, the numbers of hydrogens depends on the charge of the other atom.
Let’s take dioxygen for example:
Explaining by the common “Octect-reason” of classical Lewis Model.

Oxygen atom has 6 valence electrons(incomplete octet), so it tends to react with other atoms to fill its outermost shells. So it is unstable.
Oxygen molecule has become stable as both atoms in oxygen molecule achieve complete octet by sharing of electrons.