The Stoichiometry Calculator is a cost-free online tool that presents a balanced formula for a chemical equation. The internet stoichiometry calculator tool makes estimations much faster, and also it displays the well-balanced equation in a fraction of a second.
Table of Contents
How to Use Stoichiometry Calculator?
Below is how to use the Stoichiometry calculator:
1: Enter the chemical equation right into the input field
2: Click the “Submit” switch to get the results
3: will show the well balanced chemical formula in the new window
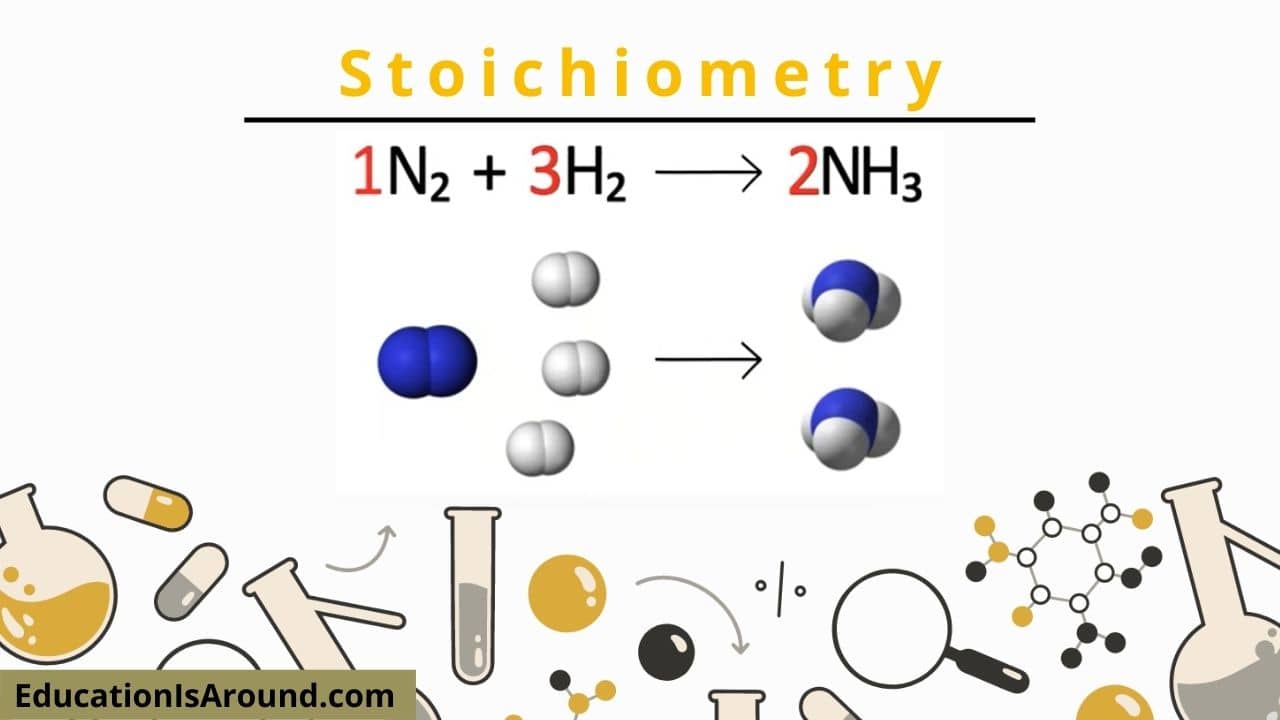
What is a Stoichiometry?
Stoichiometry is a chemistry section that uses partnerships between catalysts and items of a chain reaction to establish wanted quantitative data. Stoichiometry equates to measuring components, considering that stoikhein suggests aspect and metron implies gauge.
For stoichiometry to help run computations regarding chemical reactions, a basic understanding of the partnerships between items and catalysts and their reasons is required to learn how responses are well balanced.
Stoichiometry Calculator
What is a Chemical Formula?
To represent the various chemicals in chemistry, we use icons. It is essential to become acquainted with these basic symbols to achieve success in chemistry. For instance, the sign “C” stands for an atom of carbon, and “H” represents an atom of hydrogen.
We would make use of the notation “NaCl” to stand for a particle of common salt, sodium chloride, considering that “Na” stands for salt and also “Cl” means chlorine. In this situation, chlorine is called “chloride” due to its connection to salt. Must have covered classification or calling plans in earlier readings.
Chemical formulas are expressions of chemical processes. They are, as an example:
AgNO3 and NaCl are mixed in this equation. According to the formula, the catalysts (AgNO3 and NaCl) react via some process to form items (AgCl and also NaNO3). By undertaking a chemical reaction, they are essentially changed.
There are frequently chemical equations that show what state each compound remains in. The (s) sign indicates that the substance is potent. The (l) indicator shows that the meaning is fluid. The (aq) indicator stands for liquid in water and means the substance is dissolved in water. Lastly, the (g) indicator suggests that the importance is a gas.
In all chemical equations, coefficients are utilized to show the loved one quantities of each substance. An amount can stand for either the loved one variety of particles or the family member’s number of moles (defined below). One (1) is thought in the absence of a coefficient.
It is not uncommon for a selection of details to be composed above or below the arrowheads. By giving info such as a temperature value, we can determine what conditions are needed for a response to happen. The symbols above and listed below the arrowheads in the visuals below show that this reaction should occur at a chemical Fe2O3, a temperature level of 1000 ° C, and a stress of 500 ambiences.
The Mole: Stoichiometry Calculator
We can establish the number of moles of reactants and products from the formula above. A mole represents Avogadro’s number (6.022 x 1023) of molecules. A mole is similar to the term loads. When you have a lot of carrots, you have twelve of them. Similarly, if you have a mole of carrots, you have 6.022 x 1023 carrots. Given that there are no numbers before the terms in the formula above, each coefficient is presumed to be one (1 ). For that reason, you have the very same number of moles of AgNO3, NaCl, AgCl, and NaNO3.
It is often essential to transforming between moles and grams of a substance. The conversion can be done conveniently when the atomic and molecular mass of the substance( s) is recognized. Based on the atomic or molecular mass, a mole of that compound amounts to a gram of the mixture.
Harmonizing Chemical Equations
Nonetheless, there are times when we have to do some job before we can use the coefficients of the terms to figure out the family member number of molecules of each substance. Such holds when the formulas are not well balanced appropriately.
Because no coefficients are pointed out for any of the terms, we can think that (1) mole of Al responds with one (1) mole of Fe3O4 to produce one (1) mole of Al2O3. The reaction would be magnificent: a lightweight aluminium atom would appear out of nowhere.
We require that the variety of atoms of each element in the reactants equates to the number of bits in the products. To complete this, we have to discover each term’s relative variety of molecules as a feature of its coefficient.
Experimentation is the most reliable means to balance a straightforward chemical formula. You can determine the number of atoms in an equation in various ways, yet regardless of how you do it, you need to know how to count them. Let’s examine the copying.
More details on Stoichiometry Calculator
The term refers to 2 (2) molecules of Fe3O4. 3 (3) Fe atoms are found in each substance molecule. Consequently, in every 2 (2) molecules of the material, there must be six (6) Fe atoms. Additionally, one (1) molecule of the substance has four (4) oxygen atoms, so there must be 8 (8) oxygen atoms in 2 (2) molecules.
Establishing a technique can be challenging, but here is one way to come close to a scenario similar to this. Remember the variety of atoms on each side of the response.
The very first step to balancing is to identify a term. Considering this issue, it appears that oxygen will be the most challenging aspect of equilibrium, so we’ll begin with oxygen.
Note that the coefficient will undoubtedly provide the variety of atoms in that component. The coefficient of a reactant has a coefficient of three (3) multiplied by 4 (4 ), offering 12 oxygen atoms. The product side has a 4 (4) coefficient multiplied by a subscript of 3 (3 ), giving 12 oxygen atoms. At this moment, the oxygens have been stabilized.
Balance the equation with another term. Let’s choose iron, Fe. We include a 9 (9) coefficient in front of the Fe because there are nine (9) iron atoms in the term in which the oxygen is well balanced.
We are stabilizing the last term. On the reactant side, because we had 8 (8) aluminium atoms on the item side, we require another eight (8) before the Al term.
Restricting Reagents
The catalyst that goes out first in response to 2 or more substances occasionally happens before the other. This is called the “restricting reagent”. It is commonly required to recognize the limiting reagent in an issue.
Can solve this problem by determining how much oxygen must be if all of the catalysts have been consumed (this is the best way to obtain the maximum CO2).
We first calculate the variety of moles of C2H2 in 6.0 g of C2H2. We require a look at the periodic table to compute the moles and recognize that 1 mole of C evaluates 12.0 g, and 1 mole of H considers 1.0 g. Therefore, 1 mole of C2H2 evaluates 26 grams (2 × 12 grams + 2 × 1 gram).
To discover the complete oxygen particles, we have to increase the result by 5/2, considering that there are 5 (5) molecules of oxygen to every two (2) molecules of C2H2.
Percent Make-up
Using the per cent by mass of items or reactants in a chemical equation, it is possible to calculate the mole proportions (also referred to as mole fractions) between the terms.
There are several feasible remedies to per cent structure troubles. Increasing the answer is always an option. The proportions of CH and also C2H2 coincide. However, they are different substances. Generally, compounds are given in their most straightforward kind, where the ratio between aspects is as close to the empirical formula as feasible.
You can transform portions to grams for determining the empirical formula based on per cent structure. It is generally easier to think you have 100 grams, so 54.3% would be 54.3 grams. Having transformed the masses into moles, we can compute mole proportions. The proportions need to reduce to numbers.
Dividing all the terms by the tiniest variety of moles is an excellent technique. To create the empirical formula, the proportion of the moles is move.
Empirical Formula and Molecular Formula: Stoichiometry Calculator
An empirical formula is the most straightforward kind of a compound, whereas a molecular formula represents the term in a chemical formula. Either the empirical formula and molecular formula are the same, or the molecular formula is any positive integer multiple of the empirical formula. Completing molecular procedures are P2, C2O4, C6H14S2, H2, and C3H9.
From any substance’s mass or percentage make-up, one can compute the empirical formula. Per cent structure has already discusses above. The only thing we are doing is eliminating the action of converting portion to mass.
We need only separate the molecular mass by its empirical formula if we understand its empirical formula. The same made with among the components in the formula. Divide the mass of that aspect by one mole of the compound by its mass. If the result is a natural number, the mass proportion is proper.
Thickness
The thickness of a compound is its mass in each quantity. The term is frequently utilize in chemistry.
Concentrations of Solutions
Concentration figures out the “strength” of a remedy. Usually, an option is to dissolve something substantial in a fluid, such as salt liquifying in water. Usually, it is likewise necessary to find out how much water to add to a solution to change its focus.
Molarity
Molarity is typically utilize to describe the focus of an option. Molecularity is specifies as the number of moles of solute (what is liquifies in the remedy) divide by the quantity in litres of the solution (which contains both the liquifies compound and the treatment itself).
Molality
It is another way to gauge concentration. Molality is determine as moles of solute divide by kilos of solvent (the compound in which the solu3te is dissolve).
Final thought
A well-balanced chemical equation shows us the numerical connections between each species associated with the chemical reaction. Using these mathematical connections, we can compute mole ratios, permitting us to convert between the amounts of catalysts and products (as well as thus fix stoichiometry issues!).
Frequently asked questions
How do you determine stoichiometry?
Mostly can solve all stoichiometric troubles by complying with four basic steps:
It would help if you stabilized the equation.
Transform units of a given substance into moles.
By utilizing the mole ratio, determine the moles necessary generated by the response.
Convert moles of desired material to desired devices.
What is stoichiometry in chemistry?
Stoichiometry is precisely what it seems like. A chemical reaction is determine by the variety of moles (and, for that reason, mass) of different items and reactants. Reactions involving chemical compounds well balance or, to put it simply, have the same number of atoms in the products as in the reactants.
Why is stoichiometry so hard?
The principle of stoichiometry can be challenging because it involves a variety of personal abilities. You have to master analytical skills and prepare a problem-solving approach if you want to succeed before taking place to Determine Molar Mass, master each of these abilities
What is the mole ratio?
Among the most usual kinds of stoichiometric partnerships is the mole ratio, which explains the quantity of each compound in response to moles. Can figure out the mole ratio of a set important by analyzing the coefficients in front of each variety in a balanced chemical formula.
What is the initial step in all stoichiometry estimations?
It is vital in every stoichiometric trouble to guarantee that the chemical reaction you are managing is well balance. You recognize the idea of a ‘mole’ and the relationship between ‘amount (grams)’ and ‘moles’.





