Currently Empty: $0.00
Understanding More About Polar Covalent Bond
As a pupil of chemistry, you must understand what covalent bonds are. A lot of times, students discover it quite challenging to comprehend the principle. In such instances, it suggests that you seek help from an exclusive tutor that can clarify this to you with good reality examples. In this article, we will discuss what a polar covalent bond describes. And also illustrate it with examples.
Anybody who has observed the world to a small degree should have realized the complex frameworks. This occur from the bundling together of the primary systems of the matter referred to as atoms. The electromagnetic force binds the atoms together to produce molecular compounds. And integrated to form an even larger particle and polymer that makes existence possible.
The rising order of the complexity is made sensible. With the assistance of electro-magnetic pressures present in between the charged particles. Chemical bonding that exists at the level of atoms through the exchange or sharing of electrons. It allows the development of the molecules. The bond in between the atoms can be ionic bonding or covalent. These are both fundamental kinds of chemical bonding readily available.
Polar Covalent Bond Definition
First, it is essential to comprehend the definition of the covalent bond with a few pictures. As you are now being taught concerning covalent bonds. Let’s presume that you know what an atom and atomic framework are. This type of association is created between the atoms when sharing at least one or more pairs of electrons. These shared electrons are the valence electrons of atoms that rotate in the outermost shells.
Each atom that creates such an association attempts to obtain security by filling up the remotest digital orbital of atoms. When the two atoms get together and also share electrons, an association is produced with the help of electro-magnetic pressure of tourist attraction that exists between the nuclei and also the shared electron.
Read Also: How To Use Sohcahtoa?
The shared pairs of electrons may be equal or unequally shared based on the electro-negativity or electron fondness. When they are unequally shared, the particles end up being polar, which means that they discrepancies the fees that are developed within. The covalent bond may exist between identical or different atoms. Several of the preferred examples include Hydrogen Particle (H2), Ammonium Chloride (NH4Cl), Water (WATER), as well as Hydrogen Chloride (HCl). To get more information regarding the numerous concepts of chemistry, consider hiring an exclusive chemistry tutor today.
What Is A Polar Covalent Bond?
Throughout your secondary school or university research of the sciences, you will regularly hear water described as the perfect polar molecule and global solvent. This will undoubtedly turn up in your chemistry or organic chemistry programs, even biology as well as physics. So I suggest keeping reading to obtain a correct understanding of the beginning of this sensation.
Polar Covalent Bond Examples
Water has a chemical formula of water. It is made up of 2 atoms of hydrogen bound to an atom of oxygen. Water can additionally be referred to as dihydrogen oxide or hydrogen hydroxide.
The oxygen atom in water naturally has six valence electrons. This leaves the oxygen atom two electrons timid of fulfilling the octet guideline. The oxygen atom has two ‘selections’ to finish its octet and get to a certain valence number of 8 electrons.
Oxygen can take 2 electrons from other atoms giving it a complete octet and the official cost of adverse 2. This leaves the oxygen atom reasonably unsteady.
The second and most likely alternative is for oxygen to share two of its electrons with other atoms, providing the oxygen with a shared total of 8 electrons. In the case of the water particle, oxygen shares one electron each with two hydrogen atoms.
Read Also: Quick Summary on Lecompton Constitution
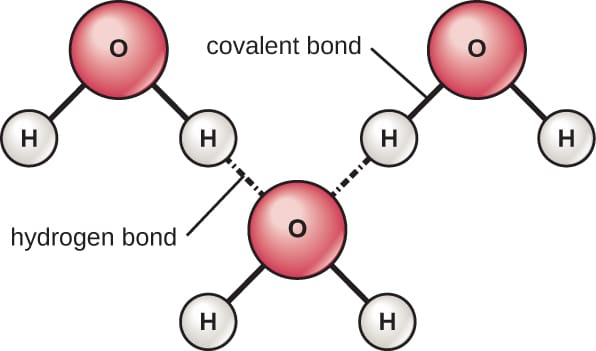
The sharing of electrons between oxygen as well as hydrogen forms a covalent bond between the atoms. Nonetheless, the sharing is not entirely equal in between both atoms.
Given that oxygen is extra electronegative, it will pull the electron density in its direction, basically ‘monopolizing’ the bonding electrons. The total electron thickness around the oxygen atom makes the oxygen partially adverse. When the electrons retreat from the hydrogen atoms, their bright nuclei come to be partly revealed. This offers the hydrogen atoms a somewhat positive charge.
Conclusion
While the general water molecule is neutral. There is now an uneven circulation of cost between the two ends of the molecule. Polarity is the phenomenon and consider there are now two polar revers on the particle.
The Partly harmful oxygen is the negative post, and the partly favourable hydrogen has a positive pole. This difference in polarity permits the water particles to draw into other particles within service. When the unfavourable end of one water molecule aligns with a different positive lot. The two particles are attract to each other. This is consider hydrogen bonding of water, and this is the very reason water is such an excellent solvent.
If a polar molecule is in water. The water molecules will certainly straighten themselves around the molecule in a comparable way. The partially adverse oxygen will indeed surround any positivity on the incoming particle, and the partly positive hydrogen atoms will line up with any negative thoughts on the dissolved particle.