Currently Empty: $0.00
What Is A Carboxyl Group
What Is A Carboxyl Group
Carboxyl Group: A carboxyl is a very common functional group seen in chemistry. A carboxyl group is defined as having a carbonyl and hydroxyl group both linked to a carbon atom. To refresh your memory, a carbonyl group is a carbon double-bonded to oxygen, and a hydroxyl group is an OH group. As a side note, don’t be fooled by the term carboxy group. This is just another way to say the carboxyl group.
A great way to make sure you know you are dealing with carboxyl is to be on the lookout for two things: an OH and a carbon double-bonded to oxygen. Better yet, if you look at the word carboxyl we can break it down into two parts: ‘carb’ and ‘oxyl.’ When you see ‘carb’ think carbon atom. When you see ‘-oxyl’ think hydroxyl group. The molecular formula for a carboxyl is COOH.
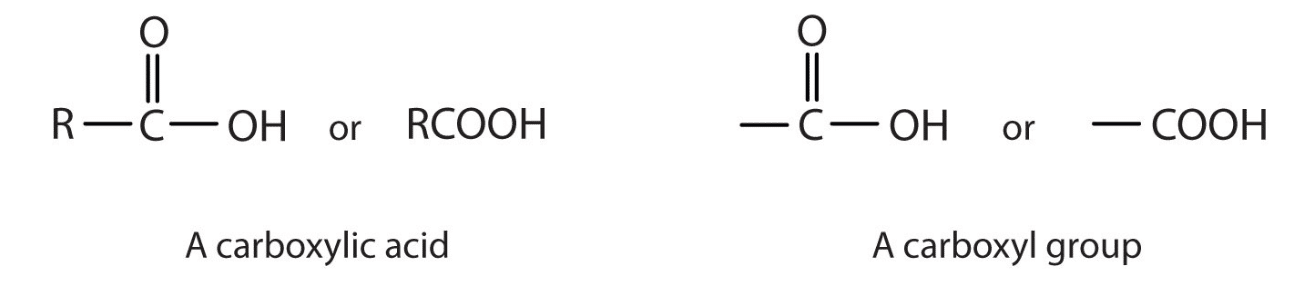
Carboxyl definition: The carboxyl is an organic functional group consisting of a carbon atom double-bonded to an oxygen atom and singly bonded to a hydroxyl group. Another way to view it is as a carbonyl group (C=O)
that has a hydroxyl group (O-H) attached to the carbon atom.
The carboxyl is commonly written as -C(=O)OH or -COOH.
Carboxyl groups ionize by releasing the hydrogen atom from the -OH group. The H+, which is a free proton, is released. Thus, carboxyl makes good acids. When hydrogen leaves, the oxygen atom has a negative charge, which it shares with the second oxygen atom in the group, allowing the carboxyl to remain stable even when oxidized.
Also known as The carboxyl is sometimes referred to as the carboxy, carboxyl functional group, or carboxyl radical.
What is the carboxyl group in organic chemistry?
A carboxyl group consists of a carbon double-bonded to oxygen and also bonded to a -OH group. Compounds with carboxyl are called carboxylic acids or organic acids. The carboxyl can act as an acid when donating a proton (H+) to a solution and becoming ionized.
Do carbohydrates have carboxyl groups?
What do carbohydrates have to do with carboxyl groups? Carbohydrates have a great number of hydroxyl (alcohol) groups: Glucose, for instance, has five. … All in all, provided the carbohydrate has a first-degree alcohol group somewhere on the molecule, it can be oxidized to form a carboxyl.
Is a carboxyl group an acid or a base?
Carboxyl Functional Group
It is one of the very important classes of organic compounds. The general formula of the class is R-C(O)OH. In this formula, R is the alkyl or aryl group. Carboxylic acids occur widely in nature. However, the majority of the members of this group are manufactured synthetically. The double bond presents in the structure of carboxylic acids play a very important role in the properties of the different compounds of carboxylic acids. Refer to the diagram below.
When a carbon compound is attached to the functional group –COOH then the compound refers to as carboxylic acids. However, the formation of a carboxyl is possible by the attachment of a hydroxyl group to a carbonyl, thus the name “carboxyl .” Carboxylic acids can be either aliphatic or aromatic on the basis of the group present. If an alkyl group is present (RCOOH) and if an aryl group is present (ArCOOH).
The higher members of the aliphatic carboxylic acids, from C12-C18, are known as fatty acids. They are found in nature as natural fats or esters of glycerol. Moreover, this group is the starting material for many essential organic compounds like esters, acid chlorides, anhydrides, amides, etc.
There are many natural compounds containing carboxylic acid. For instance formic acid is present in insect sting, butyric acid is present in butter, carbonic acid is present in the bicarbonate system of blood and tissues, lauric acid is present in coconut oil, palmitic acid is present in palm oil, arachidic acid is present in peanut oil, and stearic acid is present in chocolate, waxes, soaps, and oils. In this topic, we will discuss how the properties and structure of the carboxyl affect the properties of the compounds in the carboxylic acid group.
Carboxyl Group Definition
When you hang out with carboxyl as an organic compound, you receive the benefit of joining the club of acidity. Carboxyl will make organic compounds acidic because of their own ionizing property. Let’s dig a little deeper on this subject of carboxyl’s ionizing property.
Carboxyl will ionize themselves by letting go of the hydrogen atom on the hydroxyl. This process of ionizing themselves occurs often. When the hydrogen atom is free-floating, it is now called a free proton. It is the release of this hydrogen atom that makes a carboxyl acidic.
But what does that mean for the oxygen atom? Well, instead of remaining lonely and sad that hydrogen left, the oxygen atom will become negatively charged. As a comeback to losing his pal hydrogen, the oxygen atom will go as far as sharing this negative charge with the second oxygen atom present. By sharing a negative charge between both oxygen atoms, the carboxyl is able to remain stable while ionized.
Carboxyl StructureWait, so why is the carboxyl so willing to let the hydrogen go? The answer goes back to stability. When hydrogen is present, carboxyl is linked by a single bond to the hydroxyl and a double bond to the oxygen atom. These two different bonds make carboxyl feel uneasy as more energy is required to maintain its configuration. When hydrogen leaves, the double bond is broken, and now carboxyl is free to not only lower its energy state but also increase its stability. Hence, it makes perfect sense why carboxyl is so quick to let hydrogen go and turn into acid.
The Three Parts Of An Amino Acid Are The Amino Group
Carboxyl groups are weak acids, dissociating partially to release hydrogen ions.
The carboxyl group (symbolized as COOH) has both a carbonyl and a hydroxyl group attached to the same carbon atom, resulting in new properties.

Carboxyl groups frequently ionize, releasing the H from the hydroxyl group as a free proton (H+), with the remaining O carrying a negative charge. This charge “flip-flops” back and forth between the two oxygen atoms, which makes this ionized state relatively stable. (Hydroxyl groups sometimes ionize momentarily, but the resulting ionic forms are not stable and the ions immediately rejoin.)
Molecules containing carboxyl groups are called carboxylic acids and dissociate partially into H+ and COO−.
Carboxyl groups are common in many biological molecules, including amino acids and fatty acids.

The figure to the left illustrates acetic acid, a simple 2-carbon acid found in vinegar. Watch as the carboxyl group ionizes and the resulting ionized group is stabilized by the negative
The Carboxyl Group
The Carboxyl group is a functional organic compound. In this structure of a carboxyl group, a carbon atom is attached to an oxygen atom with the help of a double bond. It also has a single bond to a hydroxyl group. Carboxylic acids are compounds containing a carboxyl structure. There are many members in this class of organic acids such as acetic acid and amino acid.
The Carboxyl group is generally present on the sides of the molecules. The carboxyl group ionizes and releases the H atom present in the hydroxyl group part as a free H+ ion or a proton. However, the rest of the part, this is O, conveys a negative charge. The charge moves in between the two oxygen molecules forward and backward thereby making the state of ionization relatively steady.
Read Also: Spanish Verb Seguir Conjugation





